Oxidants and Antioxidants in Medical Science
Discover the potential of quantumai and revolutionize your investments.
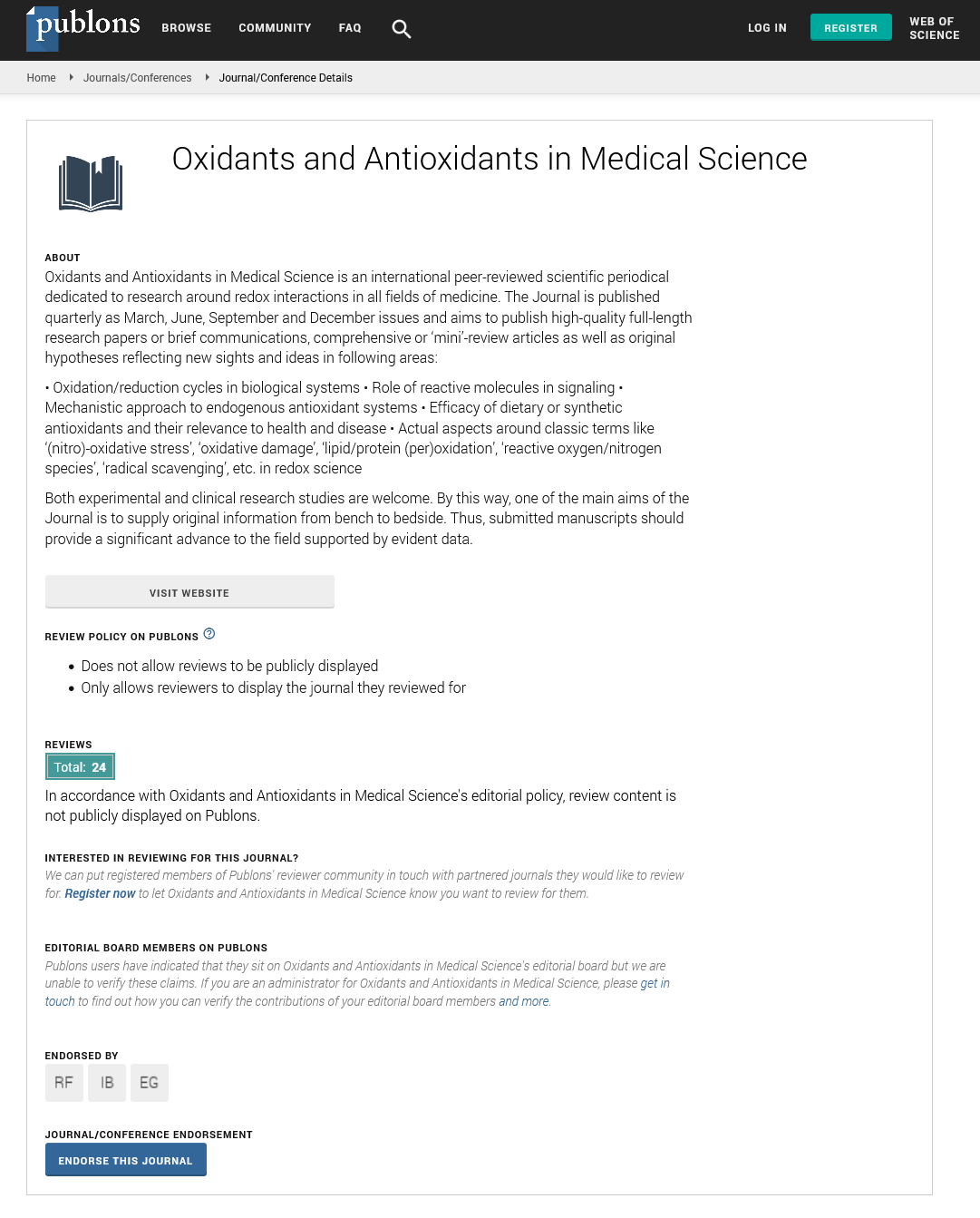
Oxidants and Antioxidants in Medical Science�is an international peer-reviewed scientific periodical dedicated to research around redox interactions in all fields of medicine. The Journal is published quarterly�and aims to publish high-quality full-length research papers or brief communications, comprehensive or �mini�-review articles as well as original hypotheses reflecting new sights and ideas in following areas:
� Oxidation/reduction cycles in biological systems
� Role of reactive molecules in signaling
� Mechanistic approach to endogenous antioxidant systems
� Efficacy of dietary or synthetic antioxidants and their relevance to health and disease
� Actual aspects around classic terms like �(nitro)-oxidative stress�, �oxidative damage�, �lipid/protein (per)oxidation�, �reactive oxygen/nitrogen species�, �radical scavenging�,�etc.�in redox science
Submit manuscript online at https://www.scholarscentral.org/submissions/oxidants-antioxidants-medical-science.html
Fast Editorial Execution and Review Process (FEE-Review Process):
Oxidants and Antioxidants in Medical Science is participating in the Fast Editorial Execution and Review Process (FEE-Review Process) with an additional prepayment of $99 apart from the regular article processing fee.�Fast Editorial Execution and Review Process is a special service for the article that enables it to get a faster response in the pre-review stage from the handling editor as well as a review from the reviewer. An author can get a faster response of pre-review maximum in 3 days since submission, and a review process by the reviewer maximum in 5 days, followed by revision/publication in 2 days. If the article gets notified for revision by the handling editor, then it will take another 5 days for external review by the previous reviewer or alternative reviewer.
Acceptance of manuscripts is driven entirely by handling editorial team considerations and independent peer-review, ensuring the highest standards are maintained no matter the route to regular peer-reviewed publication or a fast editorial review process. The handling editor and the article contributor are responsible for adhering to scientific standards. The article FEE-Review process of $99 will not be refunded even if the article is rejected or withdrawn for publication.
The corresponding author or institution/organization is responsible for making the manuscript FEE-Review Process payment. The additional FEE-Review Process payment covers the fast review processing and quick editorial decisions, and regular article publication covers the preparation in various formats for online publication, securing full-text inclusion in a number of permanent archives like HTML, XML, and PDF, and feeding to different indexing agencies.
�
2022, Volume 11, Issue 9
Commentary
-
Significant Uses and Side Effects of Lutein
Oxid Antioxid Med Sci. 2022; 11(9): 1 - 1
Opinion Article
-
Heart Palpitations due to Supplements Deficiency
Oxid Antioxid Med Sci. 2022; 11(9): 1 - 2
Commentary
-
Significant Role of Antioxidants in Eye Diseases
Oxid Antioxid Med Sci. 2022; 11(9): 1 - 1
Perspective
-
Role of Anioxidants Defense System
Oxid Antioxid Med Sci. 2022; 11(9): 1 - 2
Perspective
-
Significant Role of Antioxidants in Metabolic Syndrome
Oxid Antioxid Med Sci. 2022; 11(9): 1 - 1
2022, Volume 11, Issue 8
Perspective
-
Antioxidants, Minerals and Vitamin Supplements in Diabetes
Oxid Antioxid Med Sci. 2022; 11(8): 1 - 2
Perspective
-
Involvement of Free Radicals in Various Diseases
Oxid Antioxid Med Sci. 2022; 11(8): 1 - 2
Commentary
-
Selenium as an Antioxidant: Its Deficiencies and Toxicities
Oxid Antioxid Med Sci. 2022; 11(8): 1 - 2
Commentary
-
Significant Role of Free Radicals in Diabetes Mellitus
Oxid Antioxid Med Sci. 2022; 11(8): 1 - 2
Opinion Article
-
Significant Role of Free Radicals in Inflammation
Oxid Antioxid Med Sci. 2022; 11(8): 1 - 2
2022, Volume 11, Issue 7
Perspective
-
Causes of Glutathione Synthetase Deficiency and its Diagnosis
Oxid Antioxid Med Sci. 2022; 11(7): 1 - 2
Perspective
-
Role of Antioxidants and its Functions in Human Body
Oxid Antioxid Med Sci. 2022; 11(7): 1 - 1
Commentary
-
Important Role of Antioxidant Supplementation in Athletes
Oxid Antioxid Med Sci. 2022; 11(7): 1 - 1
Commentary
-
Measurement and Protective Functions of Glutathione
Oxid Antioxid Med Sci. 2022; 11(7): 1 - 2
Opinion Article
-
Significant Role of Antioxidants in the Treatment of Infertility
Oxid Antioxid Med Sci. 2022; 11(7): 1 - 1
2022, Volume 11, Issue 6
Commentary
-
Note on Important Health Benefits of Carotenoids
Oxid Antioxid Med Sci. 2022; 11(6): 1 - 2
Commentary
-
Lipid Peroxidation: Types and its Determination
Oxid Antioxid Med Sci. 2022; 11(6): 1 - 2
Perspective
-
Mechanisms of Action and Characteristics of Antioxidants
Oxid Antioxid Med Sci. 2022; 11(6): 1 - 1
Perspective
-
Role of Antioxidants in the Treatment of Rheumatoid Arthritis
Oxid Antioxid Med Sci. 2022; 11(6): 1 - 1
Opinion Article
-
Significant Role of Antioxidants in the Treatment of Breast Cancer
Oxid Antioxid Med Sci. 2022; 11(6): 1 - 1
2022, Volume 11, Issue 5
Perspective
-
Brief Note on Antioxidant Compounds in Eggs
Oxid Antioxid Med Sci. 2022; 11(5): 1 - 1
Perspective
-
A Brief Note on Types of DNA Damage
Oxid Antioxid Med Sci. 2022; 11(5): 1 - 1
Commentary
-
Significant Role of Antioxidants in the Treatment of Liver Disease
Oxid Antioxid Med Sci. 2022; 11(5): 1 - 1
Commentary
-
Whole Grains as an Antioxidant and its Health Benefits
Oxid Antioxid Med Sci. 2022; 11(5): 1 - 2
Opinion Article
-
Nutritional Value and Health Benefits of Popcorn
Oxid Antioxid Med Sci. 2022; 11(5): 1 - 1
2022, Volume 11, Issue 4
Perspective
-
The Important Health Benefits of Glutathione
Oxid Antioxid Med Sci. 2022; 11(4): 1 - 1
Commentary
-
Note on Oxidative Stress Related Diseases
Oxid Antioxid Med Sci. 2022; 11(4): 1 - 2
Commentary
-
Source and Roles of Reactive Nitrogen Species
Oxid Antioxid Med Sci. 2022; 11(4): 1 - 2
Perspective
-
A Brief Note on Source and Deficiency of Vitamin E
Oxid Antioxid Med Sci. 2022; 11(4): 1 - 2
Opinion Article
-
The Important Role of Antioxidants in Alzheimers Disease
Oxid Antioxid Med Sci. 2022; 11(4): 1 - 1
2022, Volume 11, Issue 3
Commentary
-
Biochemistry and Biological Source of Reactive Oxygen Species
Oxid Antioxid Med Sci. 2022; 11(3): 1 - 1
Commentry
-
A Brief Note on Source and Deficiency of Vitamin C
Oxid Antioxid Med Sci. 2022; 11(3): 1 - 1
Perspective
-
The Importance of Antioxidants in the Treatment of Ulcers
Oxid Antioxid Med Sci. 2022; 11(3): 1 - 1
Perspective
-
Redox Reactions: Types and its Importance
Oxid Antioxid Med Sci. 2022; 11(3): 1 - 1
Perspective
-
Importance of Antioxidants in Food
Oxid Antioxid Med Sci. 2022; 11(3): 1 - 1
2022, Volume 11, Issue 1
Opinion Article
-
Applications and Factors that Affect the Oxidants
Oxid Antioxid Med Sci. 2022; 11(1): 1 - 1
Perspective
-
Polyphenols and its Classification
Oxid Antioxid Med Sci. 2022; 11(1): 1 - 1
Commentary
-
Different Types of Antioxidants and its Importance
Oxid Antioxid Med Sci. 2022; 11(1): 1 - 1
Perspective
-
The Importance of Catalysis and its Classification
Oxid Antioxid Med Sci. 2022; 11(1): 1 - 1
Commentary
-
Benefits and Risk of Human Health by Antioxidants
Oxid Antioxid Med Sci. 2022; 11(1): 1 - 2
2021, Volume 10, Issue 8
Brief Report
-
Hydrogen Peroxide as an Adjuvant Therapy for COVID-19: A Case Series from Mexico
Arturo Cervantes-Trejo* and Isaac D. Castaneda
Oxid Antioxid Med Sci. 2021; 10(8): 1 - 4
Review Article
-
The Influence of Various Forms of Selenium on Redox Processes, Gene Expression of Selenoproteins, Antioxidant Status in Biological Objects
Bityutskyy VS*, Oleshko OA, Tsekhmistrenko SI, Melnichenko OM, Tsekhmistrenko OS, Melnichenko YuO, Heiko LM, Oleshko MO, Kharchyshyn VM, Fedorchenko MM, Vered PI and Shulko OP
Oxid Antioxid Med Sci. 2021; 10(8): 5 - 13
Commentary
-
Antioxidant Uses for Skin Aging and Skin Care: A Short Note
Oxid Antioxid Med Sci. 2021; 10(8): 14 - 15
Research Article
-
Effect of Mixed Aqueous Extracts of Allium Sativum, Annona Muricata and Cymbopogon
Citratus Leaves on the Blood Glucose and Lipid Profile of Hyperglycemic Rats
Ijioma Okorie*, Chiemela Goodluck Ndubuisi, Amarachi Ihedinachi Onwuchekwa, Raymond Ade Adesanmi and Ngozi Nnam
Oxid Antioxid Med Sci. 2021; 10(8): 16 - 23
Editorial
-
The Overall Health Benefits of Antioxidants Present in Red Wine
Oxid Antioxid Med Sci. 2021; 10(8): 1 - 1
2022, Volume 11, Issue 2
Commentary
-
List of Antioxidant Supplements
Oxid Antioxid Med Sci. 2022; 11(2): 1 - 1
Commentary
-
Effects of Oxidative Stress and its Prevention
Oxid Antioxid Med Sci. 2022; 11(2): 1 - 2
Perspective
-
Free Radicals: Functions, Types and its Source
Oxid Antioxid Med Sci. 2022; 11(2): 1 - 2
Perspective
-
The Importance of Antioxidants in the Treatment of Cancer
Oxid Antioxid Med Sci. 2022; 11(2): 1 - 1
Perspective
-
The Importance of Catechins
Oxid Antioxid Med Sci. 2022; 11(2): 1 - 1
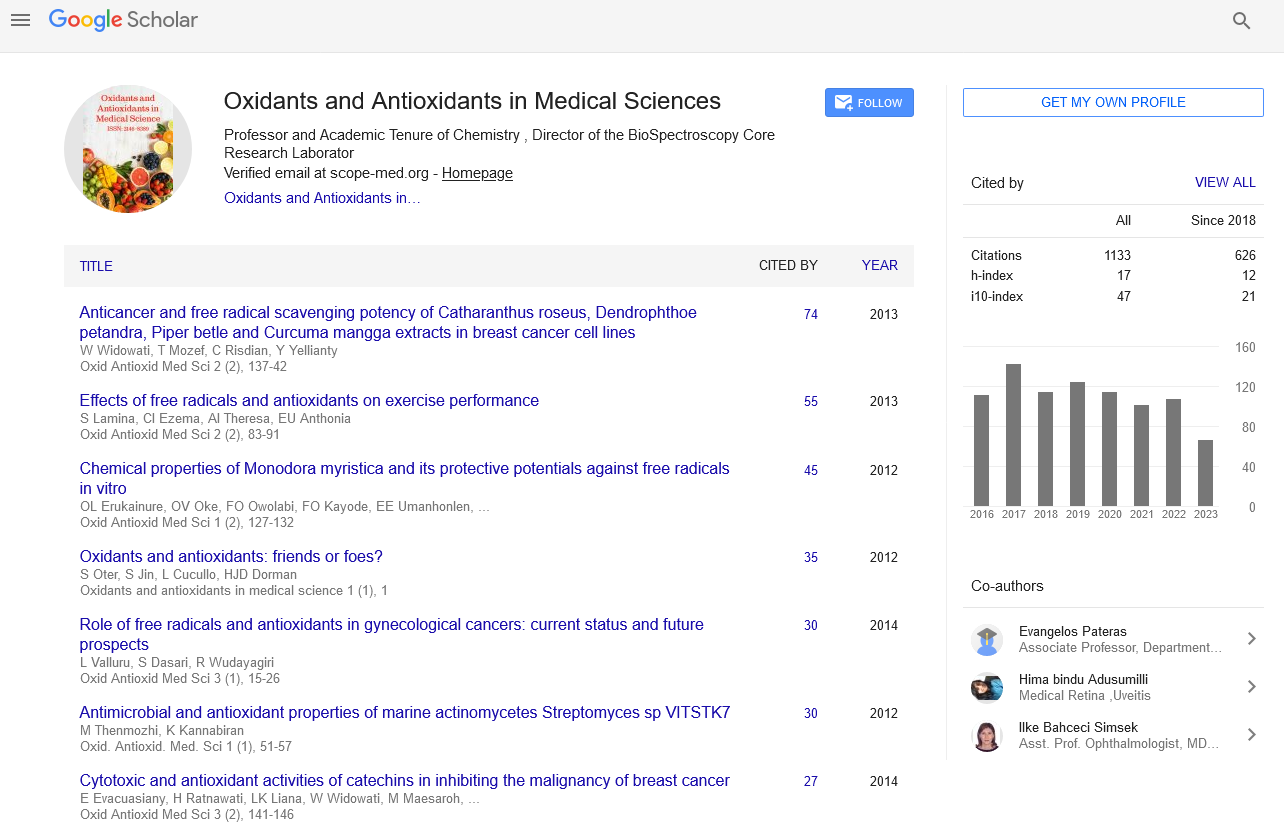
h-index
Articles published in Oxidants and Antioxidants in Medical Science have been cited by esteemed scholars and scientists all around the world. Oxidants and Antioxidants in Medical Science has got h-index 17 , which means every article in Oxidants and Antioxidants in Medical Science has got 17 average citations.